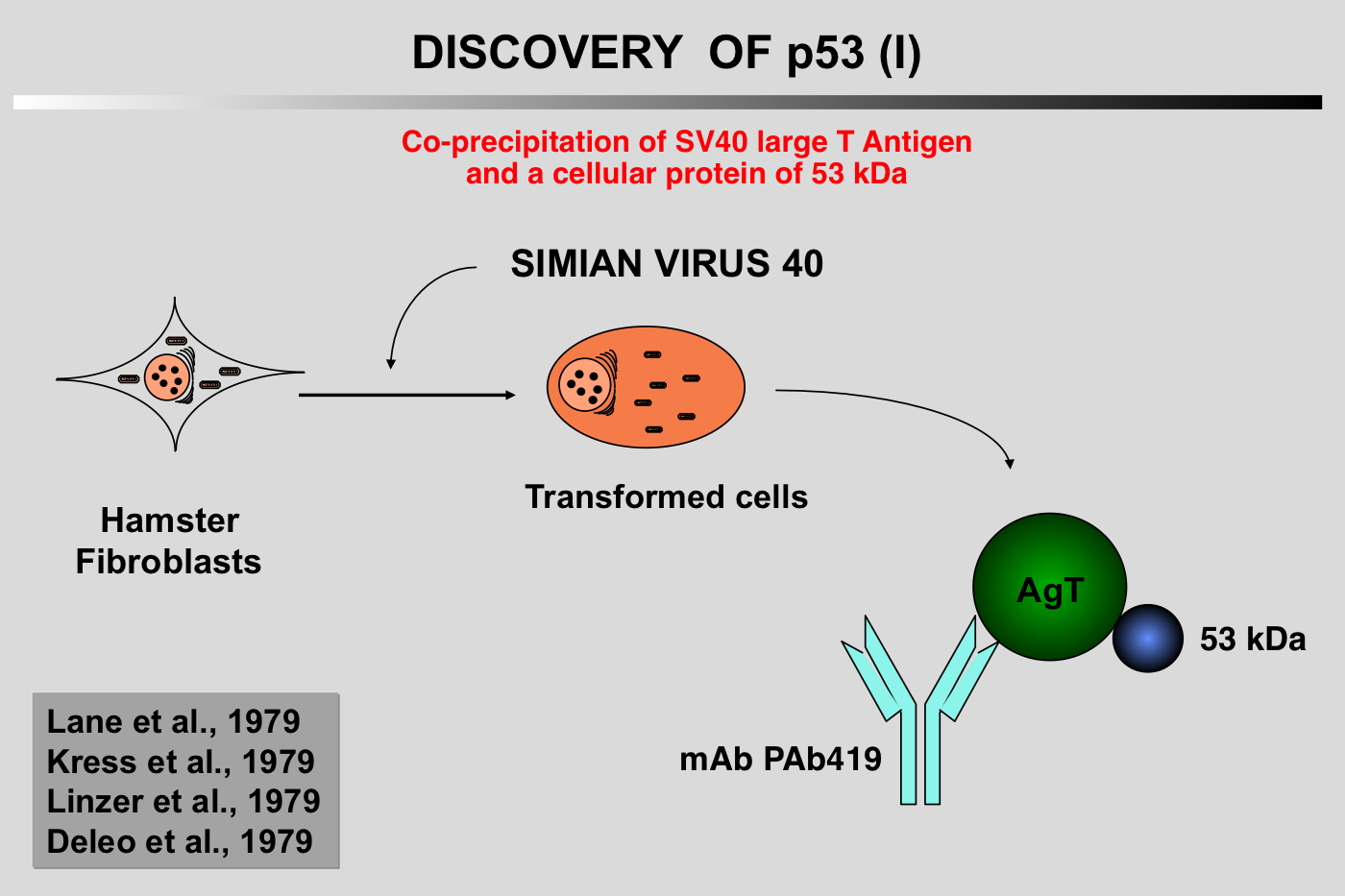
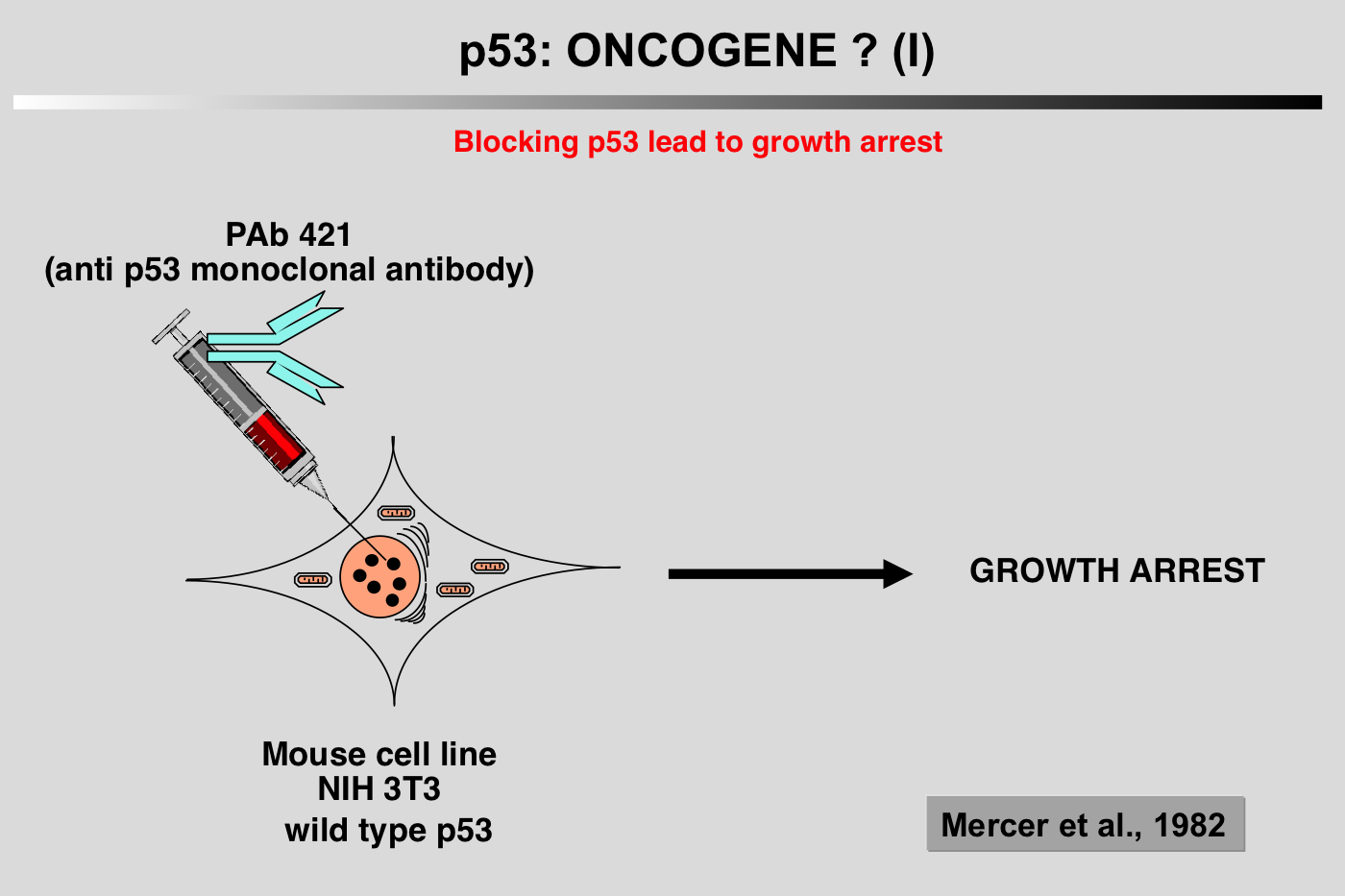
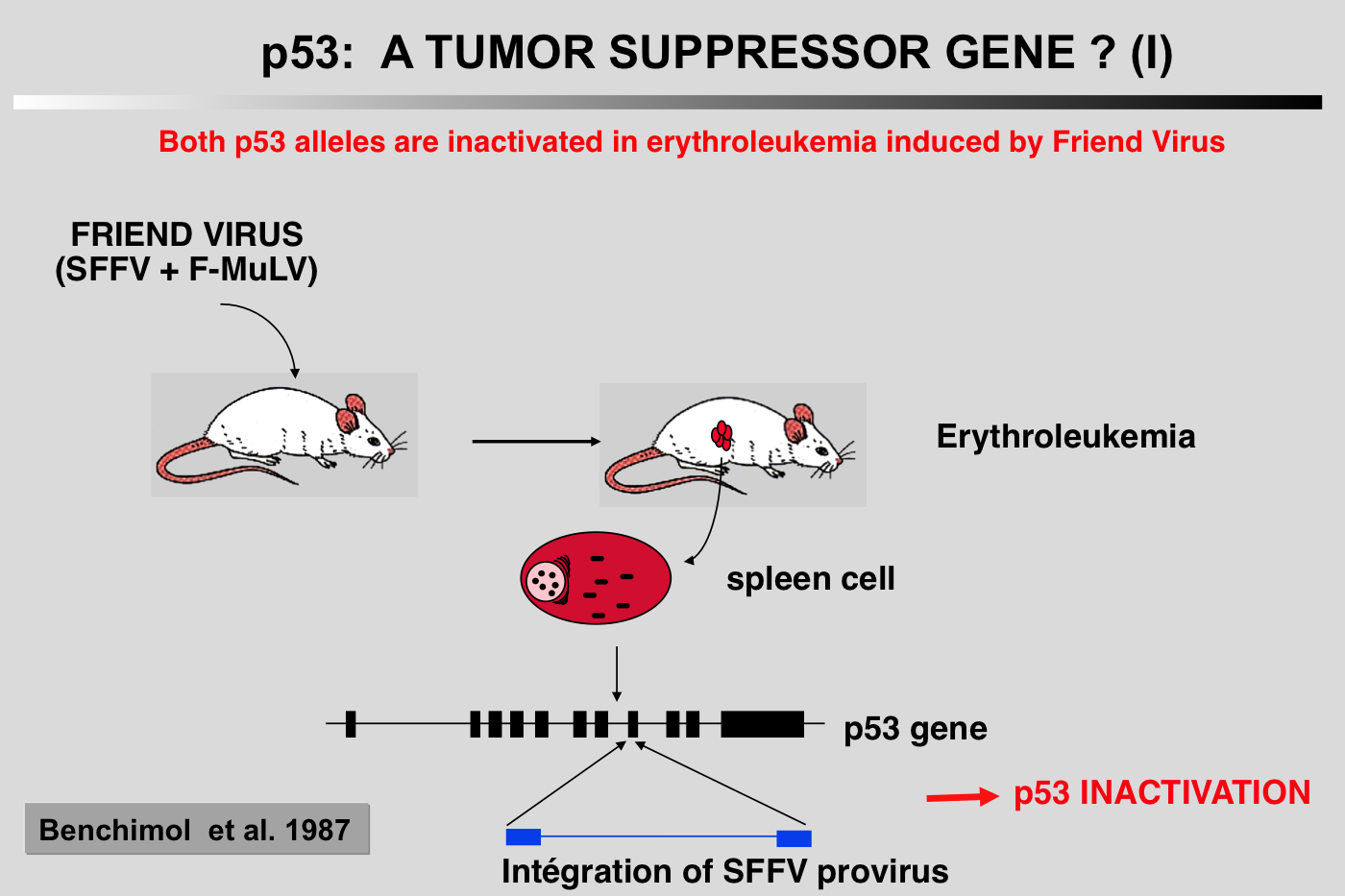
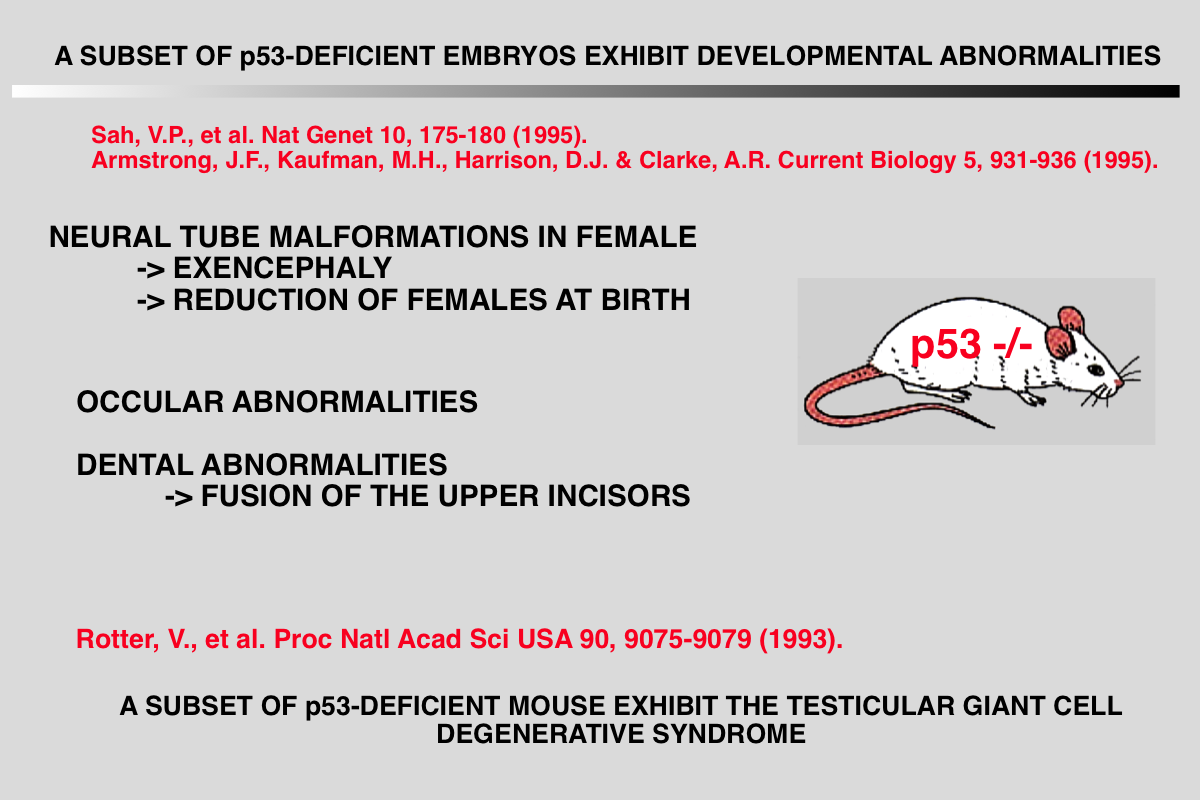
The particular characteristics of TP53 alterations, 80% of which are missense mutations leading to functionally heterogeneous proteins, make p53 a unique gene in the class of tumor suppressor genes.
A considerable number of mutant p53 proteins probably have an oncogenic activity per se and therefore actively participate in cell transformation. The fact that the apoptotic and antiproliferative functions of p53 can be dissociated in certain mutants also suggests another level of complexity in the relationships between p53 inactivation and neoplasia.
Click on the links below for further details
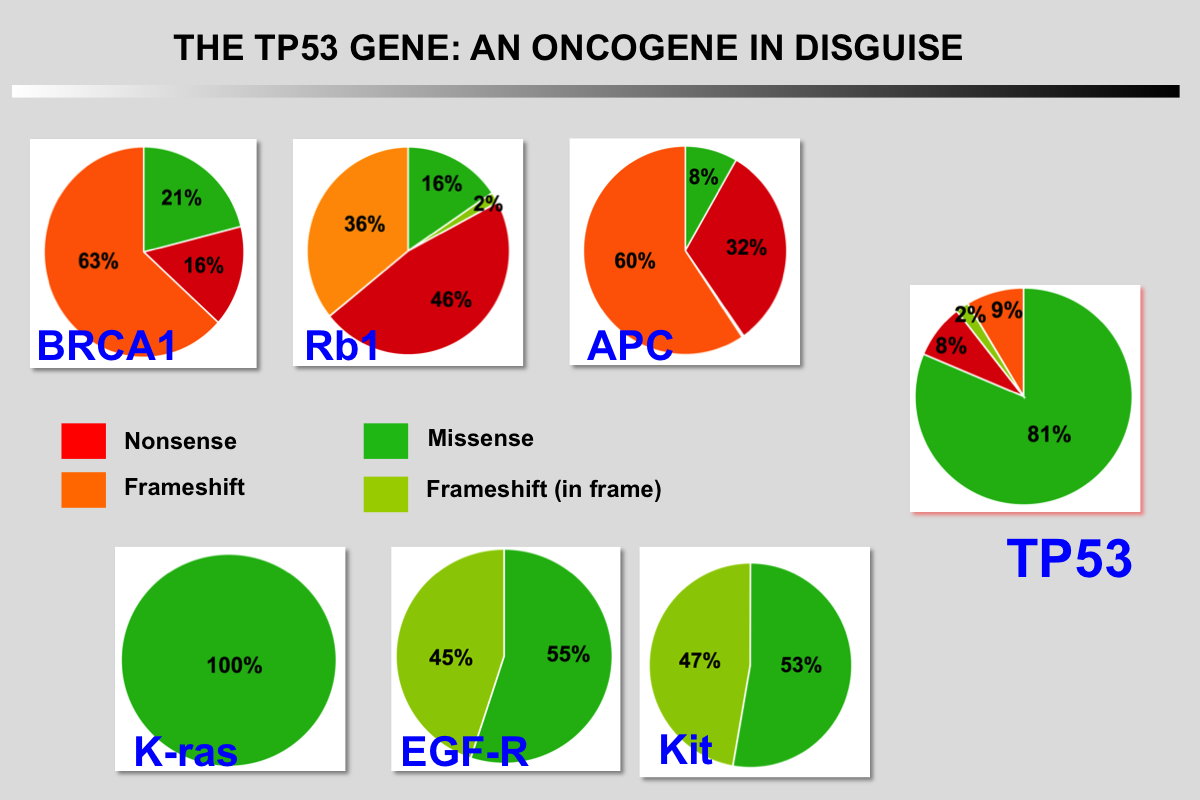
A unique feature of the TP53 gene compared to other tumor suppressor genes is its mode of inactivation. More than 80% of somatic and germline TP53 alterations are missense mutations that lead to the synthesis of a stable mutant protein that accumulates in the nucleus of tumor cells. This high frequency of substitutions is highly analogous among the various types of cancer despite a different spectrum of mutation due to variable exposure to carcinogens.
Missense mutations are highly specific to oncogenes and target specific key residues of a protein. Tumor suppressor genes display either a majority of out-of-frame insertions and deletions (indel) or nonsense mutations, both leading to a loss of protein expression. Although very crude, using these genetic events as an index to classify cancer genes has been widely used when functional consequence of the mutations were lacking.
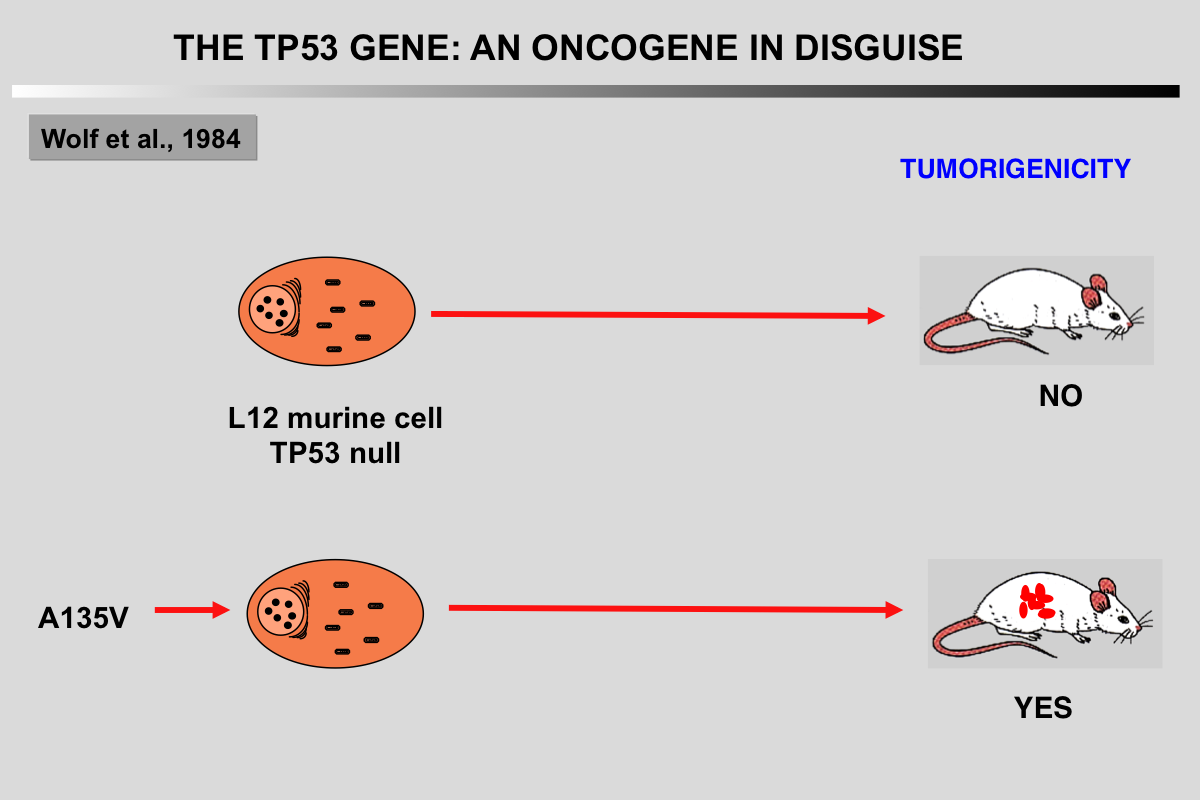
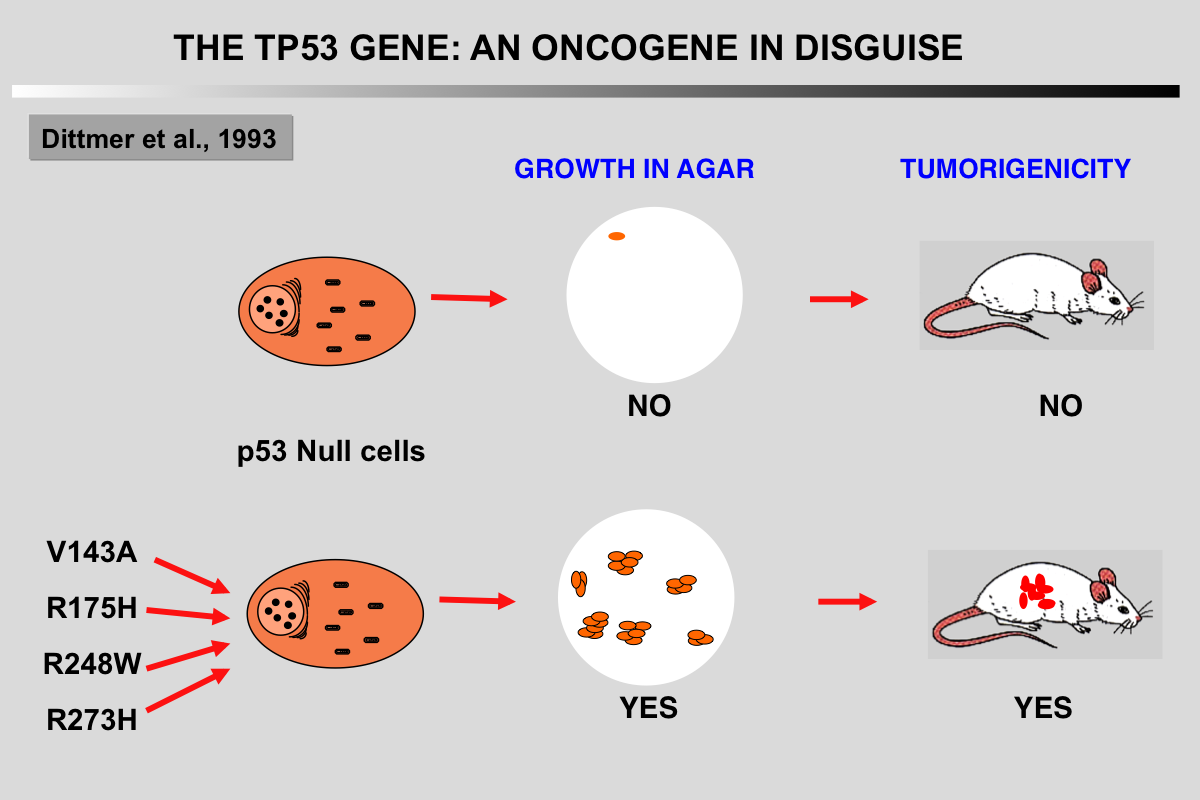
Gain of function (GOF) in mutant TP53 has been extensively investigated in multiple systems both in vitro and in vivo. GOF was hypothesized as early as 1993 when Dittmer et al. showed that the introduction of different TP53 mutants in TP53 null cells resulted in an oncogenic phenotype such as enhanced tumorigenic potential. Further studies have largely supported this observation. Mutant TP53 GOF includes enhanced tumorigenesis, metastasis, resistance to therapy and genomic instability, the latter of which found further support in a recent analysis of 3,000 tumors showing that TP53 mutations were strongly associated with a high frequency of copy number changes. GOF in TP53 mutants are highly heterogeneous, an observation that may be related to the various conformations of TP53 mutants. GOF mechanisms may be the result of changes in the specificity of the DNA binding activity of the TP53 mutant leading to the induction of novel transcriptional programs, or in changes of its interaction with other cellular proteins directly or indirectly related to the regulation of gene expression.
Gualberto, A., Aldape, K., Kozakiewicz, K., and Tlsty, T. D. (1998). An oncogenic form of p53 confers a dominant, gain-of-function phenotype that disrupts spindle checkpoint control. Proc Natl Acad Sci U S A 95, 5166-5171.
Abstract of the paper
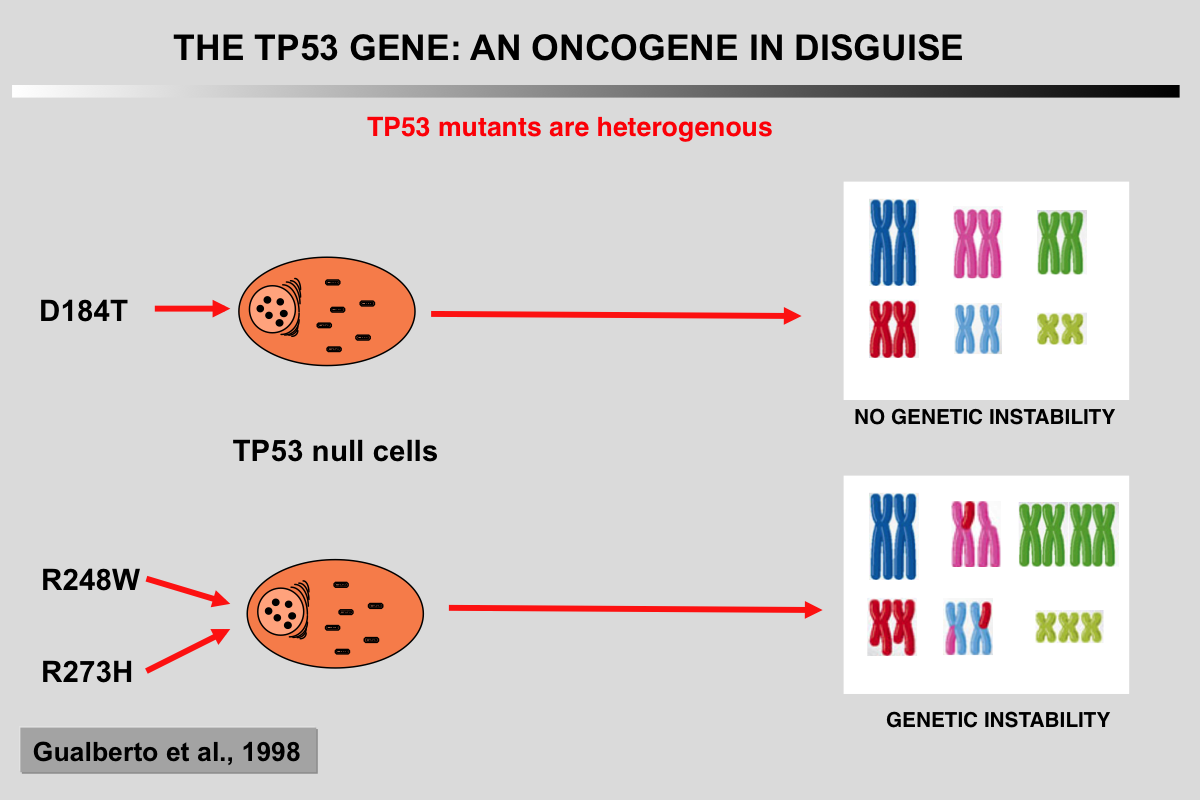
Although it is well-established that p53 functions as a tumor suppressor gene, certain mutations exhibit gain-of-function activities that increase oncogenic transformation. We have found a common class of p53 missense mutation that exhibits a dominant, gain-of-function activity that generates genomic instability. Fibroblasts from Li-Fraumeni syndrome heterozygotes with such mutations generate polyploid cells when exposed to spindle depolymerizing agents. Expression of such mutant alleles in normal fibroblasts yields the same phenotype. This class of dominant, gain-of-function p53 mutation (p53(RSC), relaxed spindle checkpoint allele) does not require the transcriptional activation function of p53 for this behavior. Thus p53 mutations can contribute to progression of a cancer cell not only by absence of p53 tumor suppressor activity but also by the presence of an activity that promotes genetic instability.
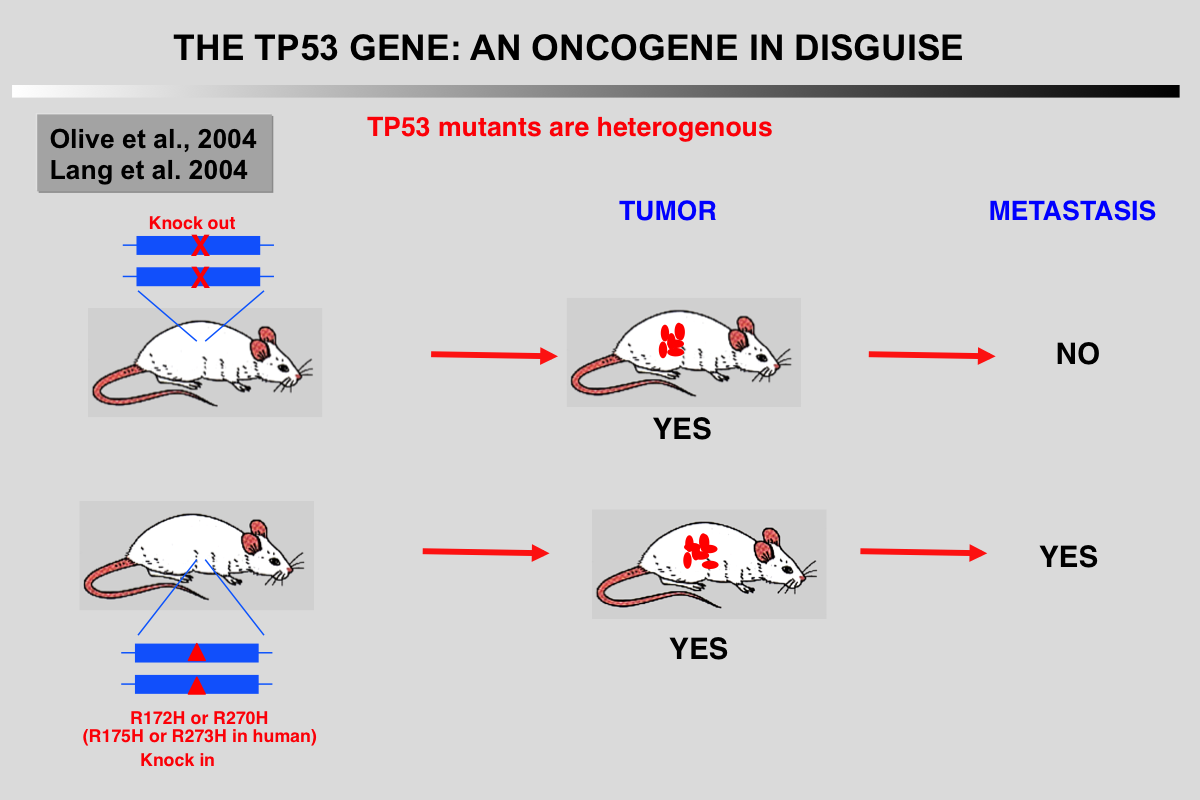
In 1992, TP53 knockout mice were generated by removing exons encoding the DNA binding domain, thus impairing the translation of all TP53 isoforms. Homozygote TP53-/- are highly prone to cancer, in particular T-cell lymphoma and sarcoma. Although these knock-out mice supported the model of TP53 as a tumor suppressor gene, they were not fully satisfactory, since in humans most cancers are carcinomas, which were observed at very low frequency in TP53 knockout mice Improvements in the production of transgenic mice led to the creation of knock-in mice expressing various hotspot mutants that include structural or DNA contact mutants and result in human-like tumors. Compared to TP53-/- mice, these novel knock-in models displayed a higher degree of heterogeneity in the spectrum of tumor types with more frequent carcinomas. Furthermore, these tumors were highly invasive and metastatic, a feature absent in TP53-/- mice.
References
Brosh, R., and Rotter, V. (2009). When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer 9, 701-713.
Dittmer, D., Pati, S., Zambetti, G., Chu, S., Teresky, A. K., Moore, M., Finlay, C., and Levine, A. J. (1993). Gain of function mutations in p53. Nat Genet 4, 42-46.
Gualberto, A., Aldape, K., Kozakiewicz, K., and Tlsty, T. D. (1998). An oncogenic form of p53 confers a dominant, gain-of-function phenotype that disrupts spindle checkpoint control. Proc Natl Acad Sci U S A 95, 5166-5171.
Lang, G. A., Iwakuma, T., Suh, Y. A., Liu, G., Rao, V. A., Parant, J. M., Valentin-Vega, Y. A., Terzian, T., Caldwell, L. C., Strong, L. C., El-Naggar, A. K., and Lozano, G. (2004). Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell 119, 861-872.
Olive, K. P., Tuveson, D. A., Ruhe, Z. C., Yin, B., Willis, N. A., Bronson, R. T., Crowley, D., and Jacks, T. (2004). Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell 119, 847-860.
Leroy, B., Anderson, M., and Soussi, T. (2014). TP53 Mutations in Human Cancer: Database Reassessment and Prospects for the Next Decade. Hum Mutat 35, 672-688.
Bisio, A., Ciribilli, Y., Fronza, G., Inga, A., and Monti, P. (2014). TP53 Mutants in the Tower of Babel of Cancer Progression. Hum Mutat 35, 689-701.