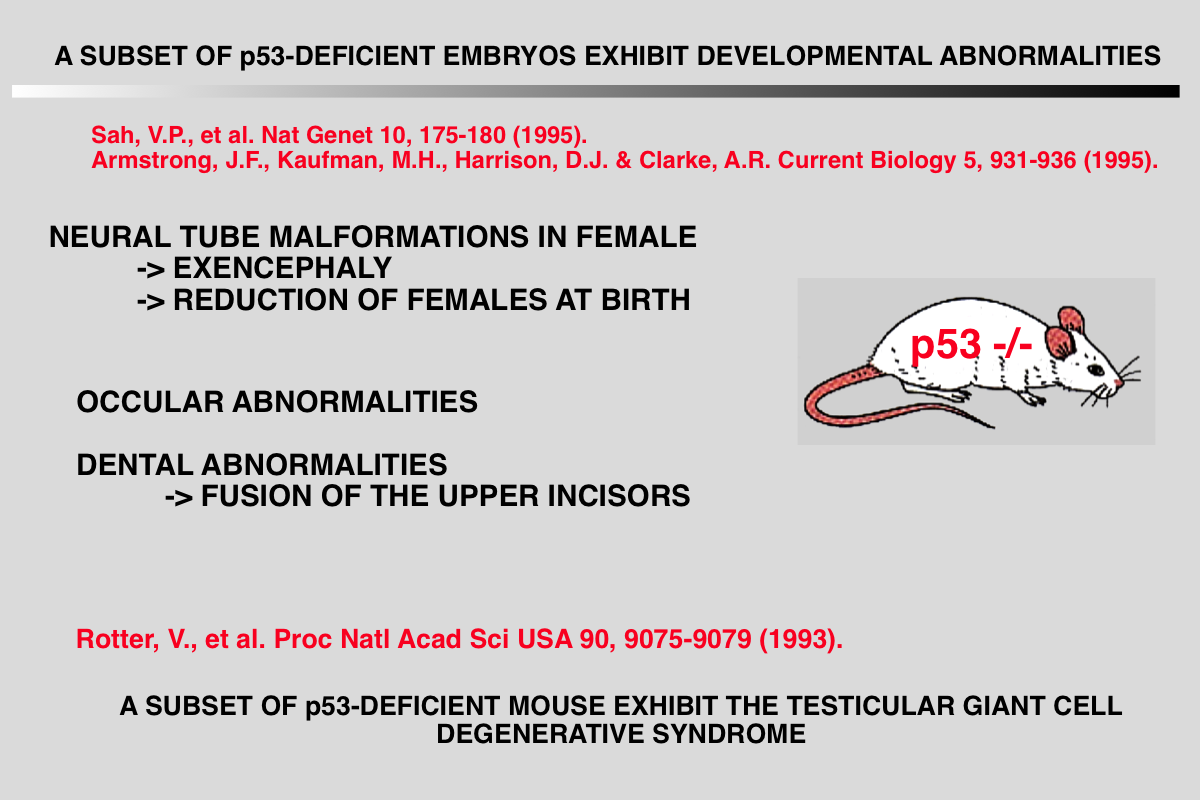
References
Calabretta B, Kaczmarek LL, Selleri L, Torelli G, Ming PML, Ming SC and Mercer WE (1986) Growth-dependent expression of human Mr 53,000 tumor antigen messenger RNA in normal and neoplastic cells. Cancer. Res. 46: 5738-5742.
Deppert W, Buschhausendenker G, Patschinsky T and Steinmeyer K (1990) Cell cycle control of p53 in normal (3T3) and chemically transformed (Meth-A) mouse cells . II. requirement for cell cycle progression. Oncogene 5: 1701-1706.
Eliyahu D, Raz A, Gruss P, Givol D and Oren M (1984) Participation of p53 cellular tumour antigen in transformation of normal embryonic cells. Nature 312: 646-649.
Jenkins JR, Rudge K, Chumakov P and Currie GA (1985) The cellular oncogene p53 can be activated by mutagenesis. Nature 317: 816-818.
Jenkins JR, Rudge K and Currie GA (1984) Cellular immortalization by a cDNA clone encoding the transformation-associated phosphoprotein p53. Nature 312: 651-654.
Mercer WE, Avignolo C and Baserga R (1984) Role of the p53 protein in cell proliferation as studied by microinjection of monoclonal antibodies. Mol. Cell. Biol. 4: 276-281.
Mercer WE, Nelson D, DeLeo AB, Old J and Baserga R (1982) Microinjection of monoclonal antibody to protein p53 inhibits serum-induced DNA synthesis in 3T3 cells. Proc Natl Acad Sci USA 79: 6309-6312.
Milner J and McCornick F (1980) Lymphocyte stimulation: concanavalin A induces the expression of a 53k protein. Cell. Biol. Int. Rep. 4: 663-667.
Parada LF, Land H, Weinberg RA, Wolf D and Rotter W (1984) Cooperation between gene encoding p53 tumour antigen and ras in cellular transformation. Nature 312: 649-651.
Reich NC and Levine AJ (1984) Growth regulation of a cellular tumour antigen, p53, in non transformed cells. Nature 308: 199-201.
Shohat O, Greenberg M, Reisman D, Oren M and Rotter V (1987) Inhibition of cell growth mediated by plasmids encoding p53 anti-sense. Oncogene 1: 277-283.
Steinmeyer K, Maacke H and Deppert W (1990) Cell cycle control by p53 in normal (3T3) and chemically transformed (meth-A) mouse cells . I. regulation of p53 expression. Oncogene 5: 1691-1699.


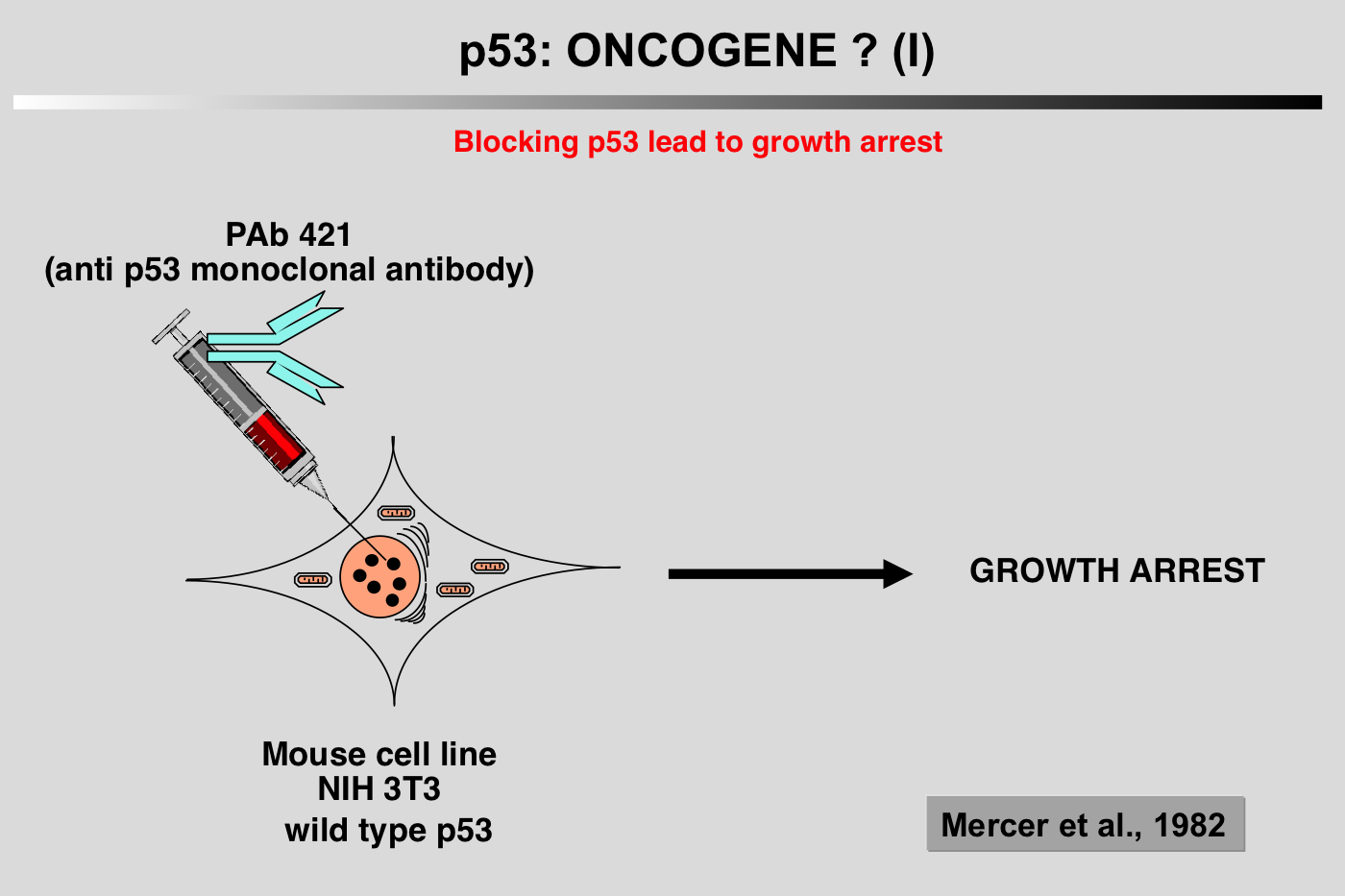 Several characteristics of the p53 protein (short half-life, nuclear localization) led to the notion that wild-type p53 could play a positive role in cell proliferation. This idea was supported by the work of Mercer and coworkers (Mercer et al. 1984; Mercer et al. 1982). Microinjection of p53 antibody (200.47 and PAb122) into the nucleus of quiescent Swiss 3T3 mouse cells inhibited the subsequent entry of the cell into the S phase after serum stimulation. This inhibition was effective only when microinjection was performed at or around the time of growth stimulation, suggesting that p53 is critical for G0/G1 transition (Mercer et al. 1984; Mercer et al. 1982).
Several characteristics of the p53 protein (short half-life, nuclear localization) led to the notion that wild-type p53 could play a positive role in cell proliferation. This idea was supported by the work of Mercer and coworkers (Mercer et al. 1984; Mercer et al. 1982). Microinjection of p53 antibody (200.47 and PAb122) into the nucleus of quiescent Swiss 3T3 mouse cells inhibited the subsequent entry of the cell into the S phase after serum stimulation. This inhibition was effective only when microinjection was performed at or around the time of growth stimulation, suggesting that p53 is critical for G0/G1 transition (Mercer et al. 1984; Mercer et al. 1982).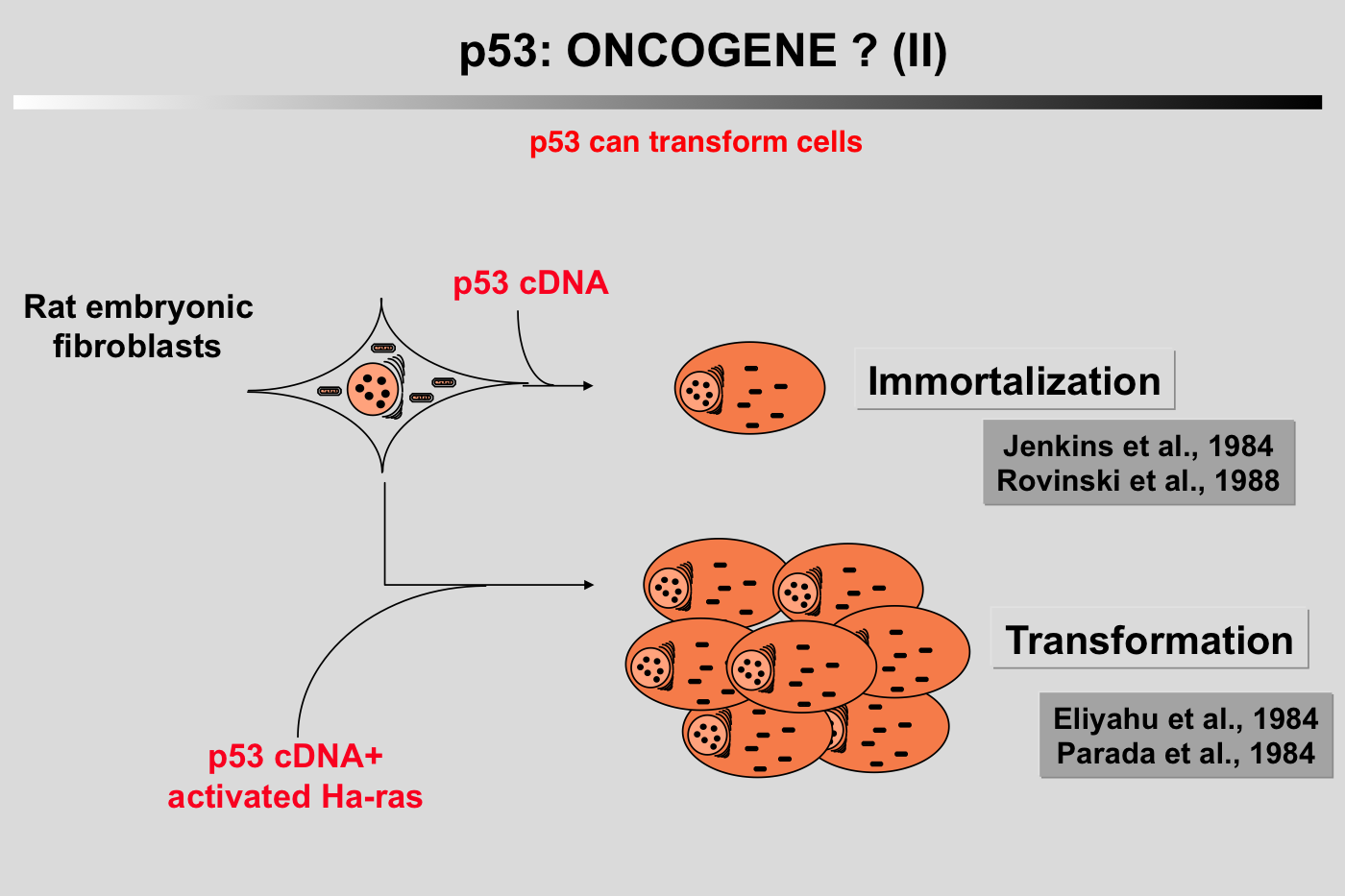 In 1984, two groups reported that cotransfection of murine p53 with plasmids encoding an activated c-Ha-ras oncogene could transform REF cells in a similar way to that observed with proto-oncogenes such as myc or E1A (Eliyahu et al. 1984; Parada et al. 1984).
In 1984, two groups reported that cotransfection of murine p53 with plasmids encoding an activated c-Ha-ras oncogene could transform REF cells in a similar way to that observed with proto-oncogenes such as myc or E1A (Eliyahu et al. 1984; Parada et al. 1984).