Absence of TP53 leads to developmental defects as well as poor fertility in female mice. Whether this phenotype is related to the tumor supressive function of TP53 remains to be defined.
Click on the links below for further details
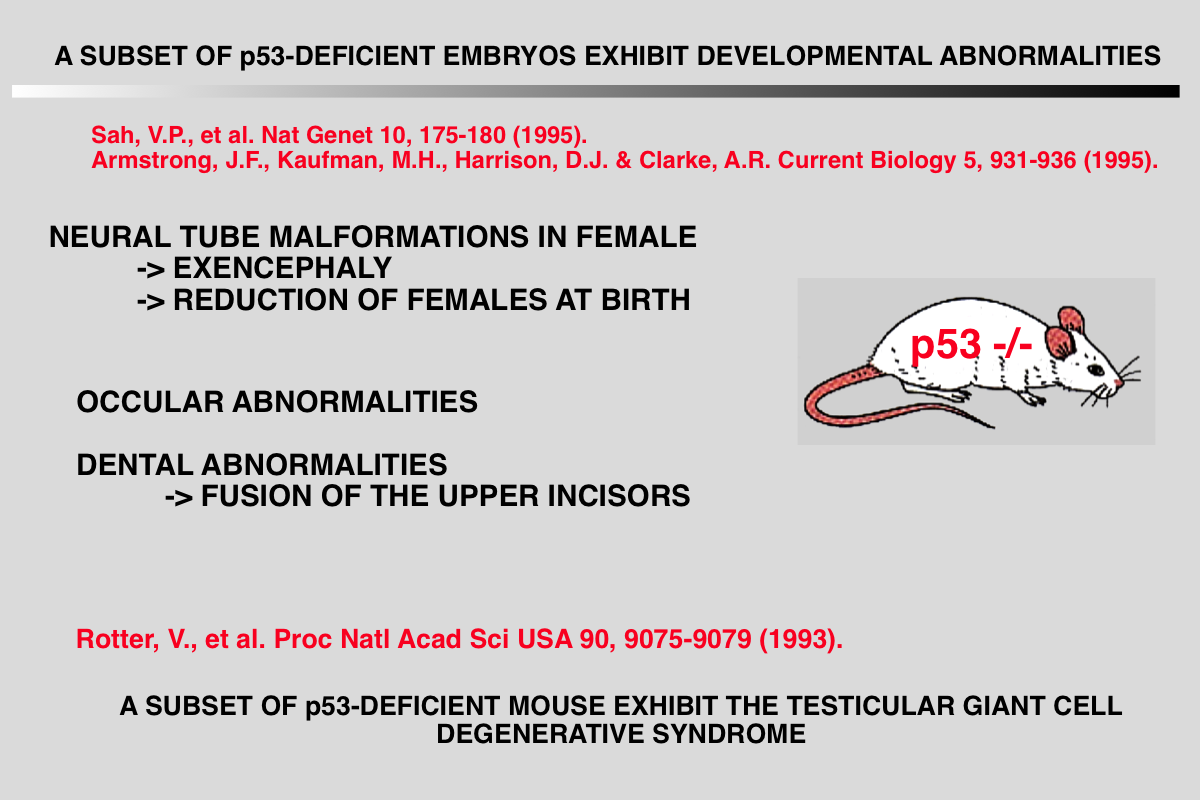
Sah VP, Attardi LD, Mulligan GJ, Williams BO, Bronson RT and Jacks T (1995) A subset of p53-deficient embryos exhibit exencephaly. Nat Genet 10: 175-80.
Abstract of the paper
BACKGROUND: Several strains of mice carrying null mutations of the tumour suppressor gene p53 have been developed. It has been reported that homozygous mice from all of these strains develop normally to birth, but then succumb rapidly to neoplasia. RESULTS: Here, we report that a significant proportion of female p53-/- mice die during embryogenesis or in the period between birth and weaning, being subject to a spectrum of abnormalities. In a significant proportion (23%) of p53-/- female embryos, the normal process of neural tube closure failed, leading to exencephaly and subsequent anencephaly. Although this phenomenon was predominantly associated with females, we observed one affected male embryo. In addition to a spectrum of neural tube defects, many of these embryos exhibited a range of craniofacial malformations, including ocular abnormalities and defects in upper incisor tooth formation. We observed a significant reduction in the number of p53-/- female progeny of p53+/- x p53+/- matings, and also in an in utero analysis of the p53+/- female progeny of p53-/- x p53+/+ matings. When male mice were exposed to irradiation prior to mating, a significant increase in the rate of abnormality was seen in the progeny, which was specifically associated with p53 deficiency. CONCLUSIONS: We have identified a high rate of developmental abnormalities associated with p53 deficiency. This manifests itself as a spectrum of lesions, predominantly female-associated defects in neural tube closure. These defects may arise either because p53 plays a physiological role at the time of neural tube closure, or because of an abnormally high frequency of mutation within the haploid gametes of p53- null parents.
Rotter V, Schwartz D, Almon E, Goldfinger N, Kapon A, Meshorer A, Donehower LA and Levine AJ (1993) Mice with reduced levels of p53 protein exhibit the testicular giant- cell degenerative syndrome. Proc Natl Acad Sci U S A 90: 9075-9.
Abstract of the paper
Transgenic mice which carry hybrid p53 promoter-chloramphenicol acetyltransferase (CAT) transgenes were found to express CAT enzymatic activity predominantly in the testes. Endogenous levels of p53 mRNA and protein were lower than in the nontransgenic control mice. The various p53 promoter-CAT transgenic mice exhibited in their testes multinucleated giant cells, a degenerative syndrome resulting presumably from the inability of the tetraploid primary spermatocytes to complete meiotic division. The giant-cell degenerative syndrome was also observed in some genetic strains of homozygous p53 null mice. In view of the hypothesis that p53 plays a role in DNA repair mechanisms, it is tempting to speculate that the physiological function of p53 that is specifically expressed in the meiotic pachytene phase of spermatogenesis is to allow adequate time for the DNA reshuffling and repair events which occur at this phase to be properly completed. Primary spermatocytes which have reduced p53 levels are probably impaired with respect to DNA repair, thus leading to the development of genetically defective giant cells that do not mature
Armstrong JF, Kaufman MH, Harrison DJ and Clarke AR (1995) High-frequency developmental abnormalities in p53-deficient mice. Curr Biol 5: 931-6.
Abstract of the paper
Defects in neural tube formation are among the most common malformations leading to infant mortality. Although numerous genetic loci appear to contribute to the defects observed in humans and in animal model systems, few of the genes involved have been characterized at the molecular level. Mice lacking the p53 tumour suppressor gene are predisposed to tumours, but the viability of these animals indicates that p53 function is not essential for embryonic development. Here, we demonstrate that a fraction of p53-deficient embryos in fact do not develop normally. These animals display defects in neural tube closure resulting in an overgrowth of neural tissue in the region of the mid- brain, a condition known as exencephaly
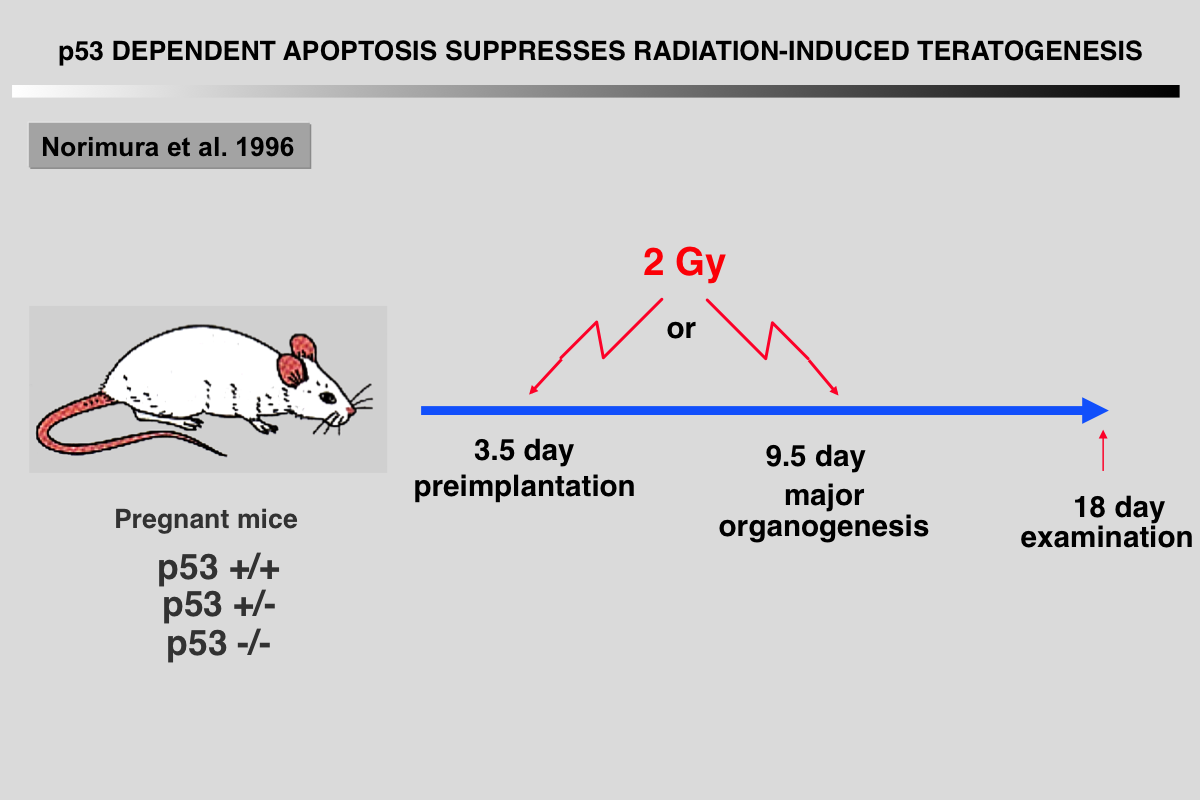
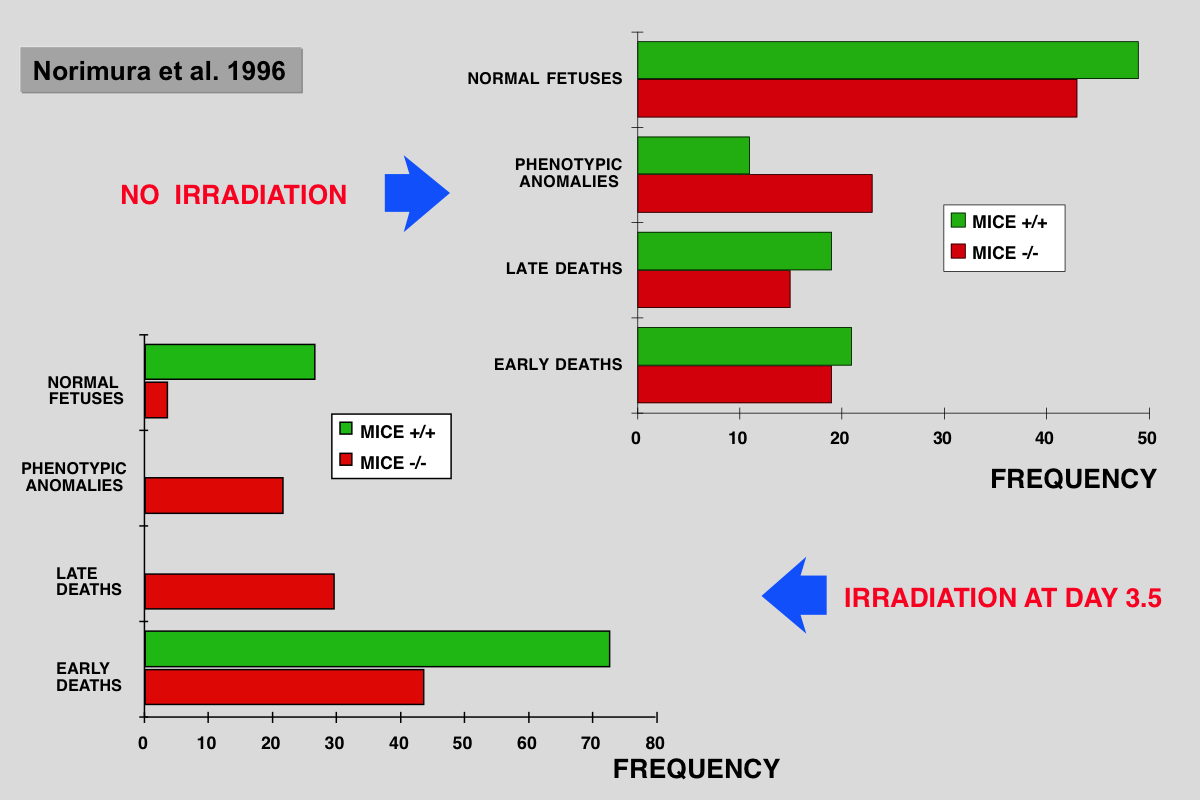
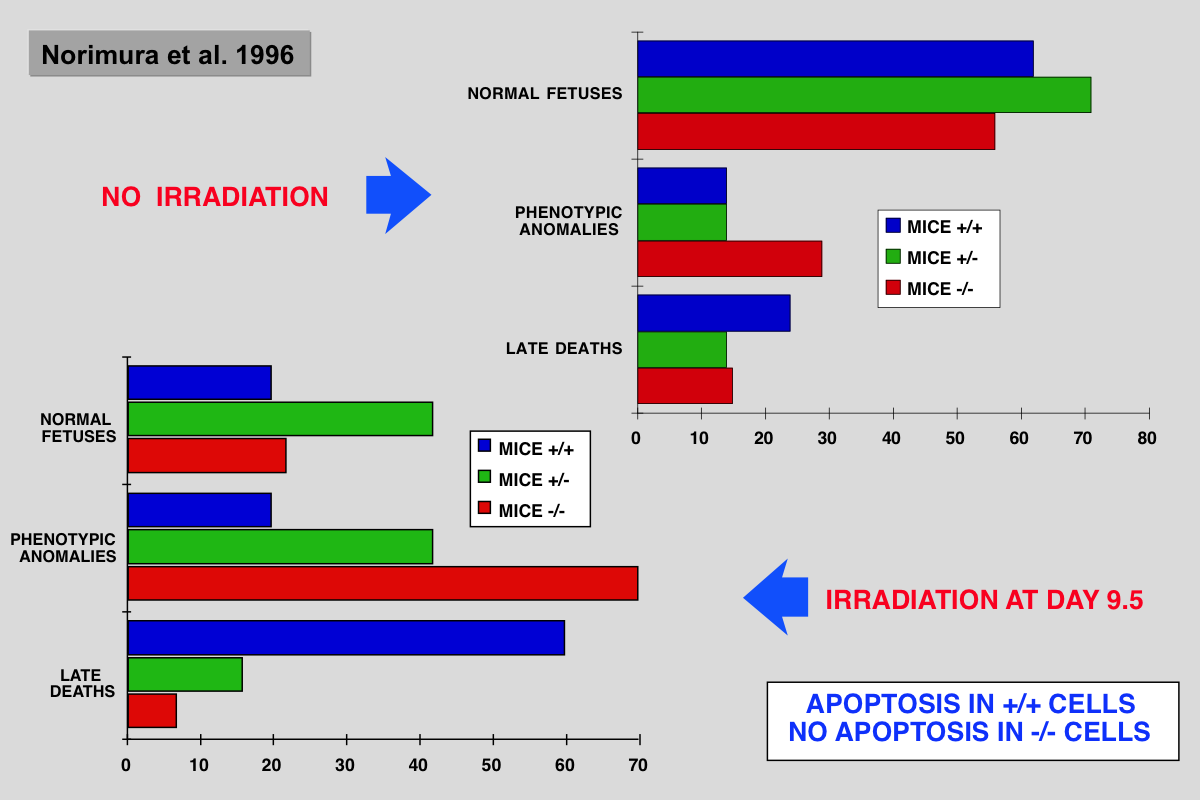
Norimura T, Nomoto S, Katsuki M, Gondo Y and Kondo S (1996) p53-dependent apoptosis suppresses radiation-induced teratogenesis. Nature Med 2: 577-580.
Abstract of the paper
About half of human conceptions are estimated not to be implanted in the uterus, resulting in unrecognizable spontaneous abortions(1,2), and about 5% of human births have a recognizable malformation(1,3). In order to find clues to the mechanisms of malformation and abortion, we compared the incidences of radiation-induced malformations and abortions in p53 null (p53(-/-)) and wild-type (p53(+/+)) mice. After X-irradiation with 2 Cy on day 9.5 of gestation, p53(-/-) mice showed a 70% incidence of anomalies and a 7% incidence of deaths, whereas p53(+/+) mice had a 20% incidence of anomalies and a 60% incidence of deaths. Similar results were obtained after irradiation on day 3.5 of gestation. This reciprocal relationship of radiosensitivity to anomalies and to embryonic or fetal lethality supports the notion that embryonic or fetal tissues have a p53-dependent ''guardian'' of the tissue(4) that aborts cells bearing radiation-induced teratogenic DNAdamage. In fact, after X-irradiation, the number of cells with apoptotic DNA fragments was greatly increased in tissues of the p53(+/+) fetuses but not in those of the p53(-/-) fetuses
.
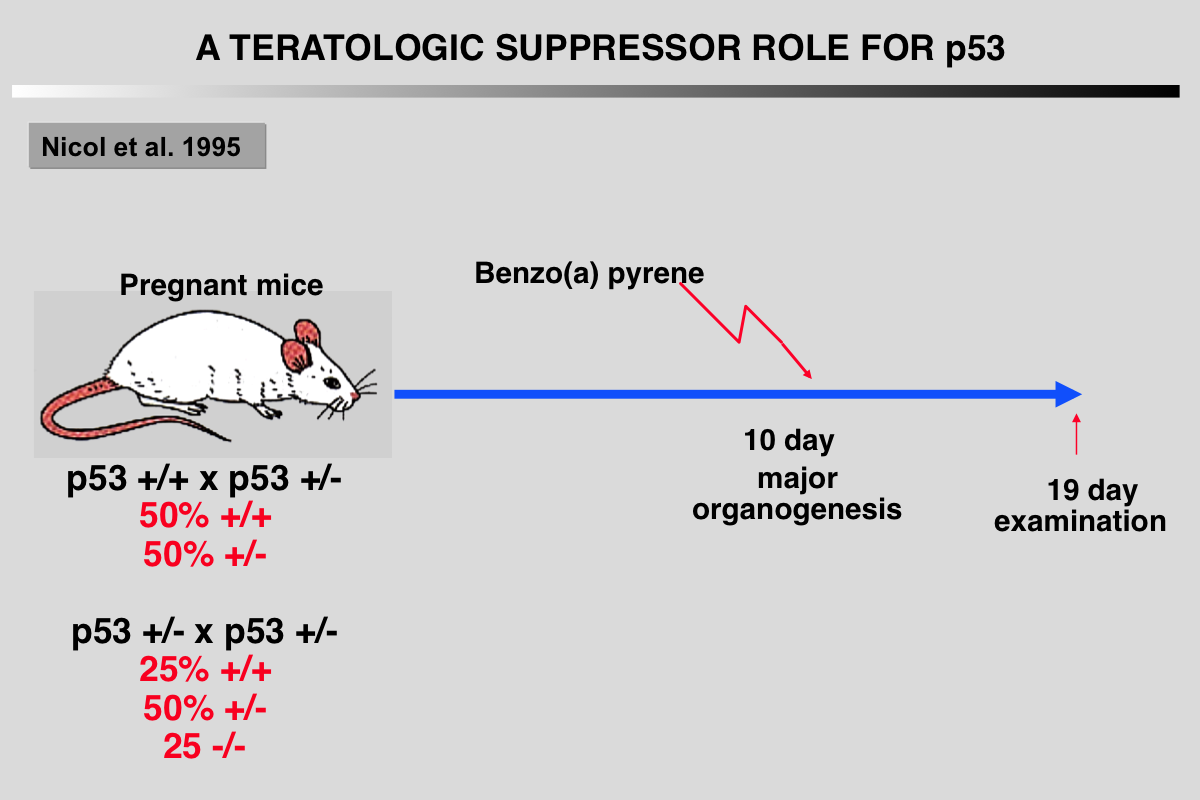
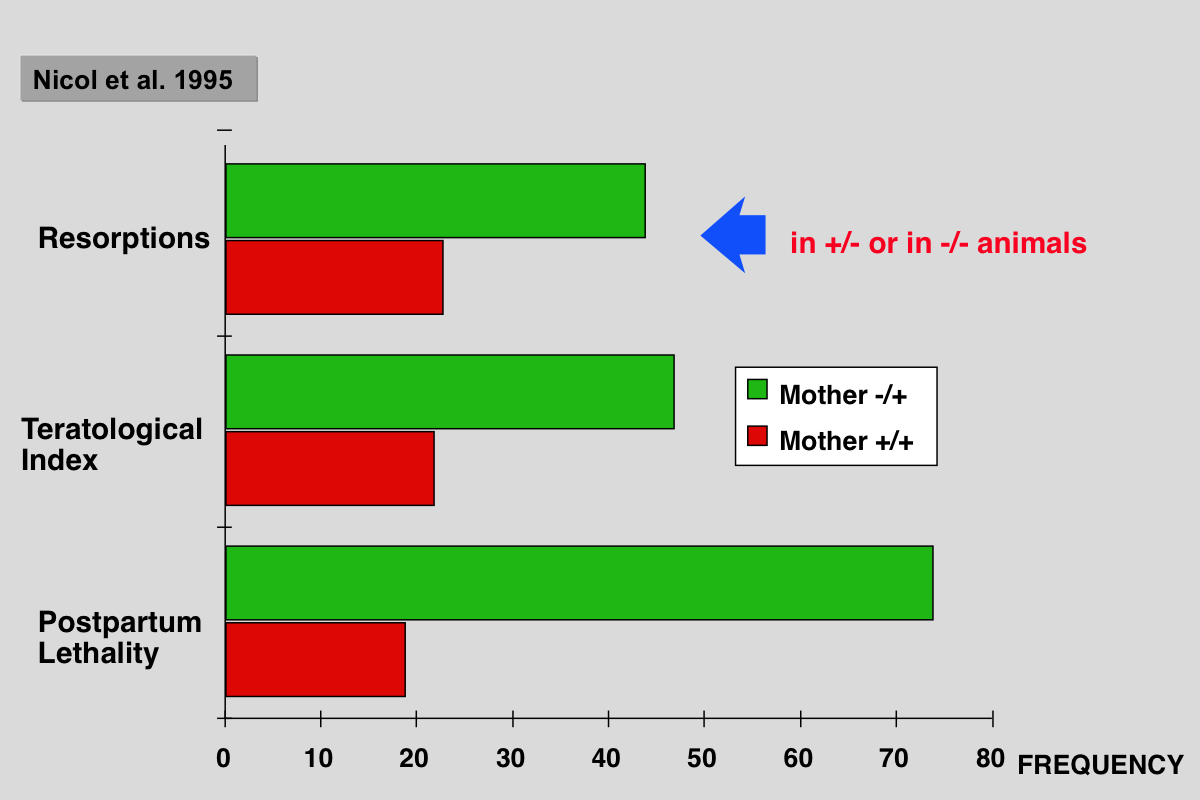
Nicol CJ, Harrison ML, Laposa RR, Gimelshtein IL and Wells PG (1995) A teratologic suppressor role for p53 in benzo[a]pyrene-treated transgenic p53-deficient mice. Nat Genet 10: 181-187.
Abstract of the paper
DNA damage may mediate birth defects caused by many drugs and environmental chemicals, therefore p53, a tumour suppressor gene that facilitates DNA repair, may be critically embryoprotective. We have studied the effects of the environmental teratogen, benzo[a]pyrene, on pregnant heterozygous p53-deficient mice. Such mice exhibited between 2- to 4-fold higher embryotoxicity and teratogenicity than normal p53- controls. Fetal resorptions reflecting in utero death were genotyped using the polymerase chain reaction and found to be increased 2.6-fold and 3.6-fold respectively with heterozygous and homozygous p53- deficient embryos. These results provide the first direct evidence that p53 may be an important teratological suppressor gene which protects the embryo from DNA-damaging chemicals and developmental oxidative stress.
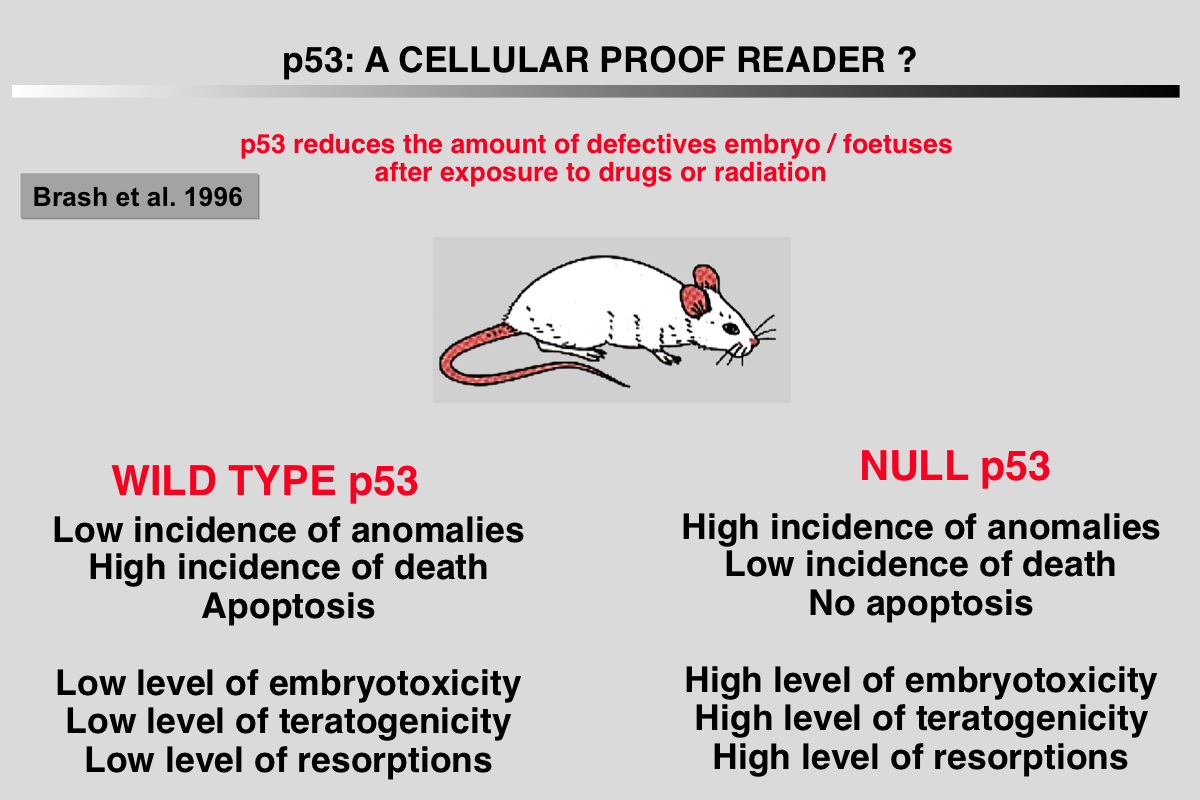
Brash, D. E. (1996). Cellular proofreading. Nat Med 2, 525-526.
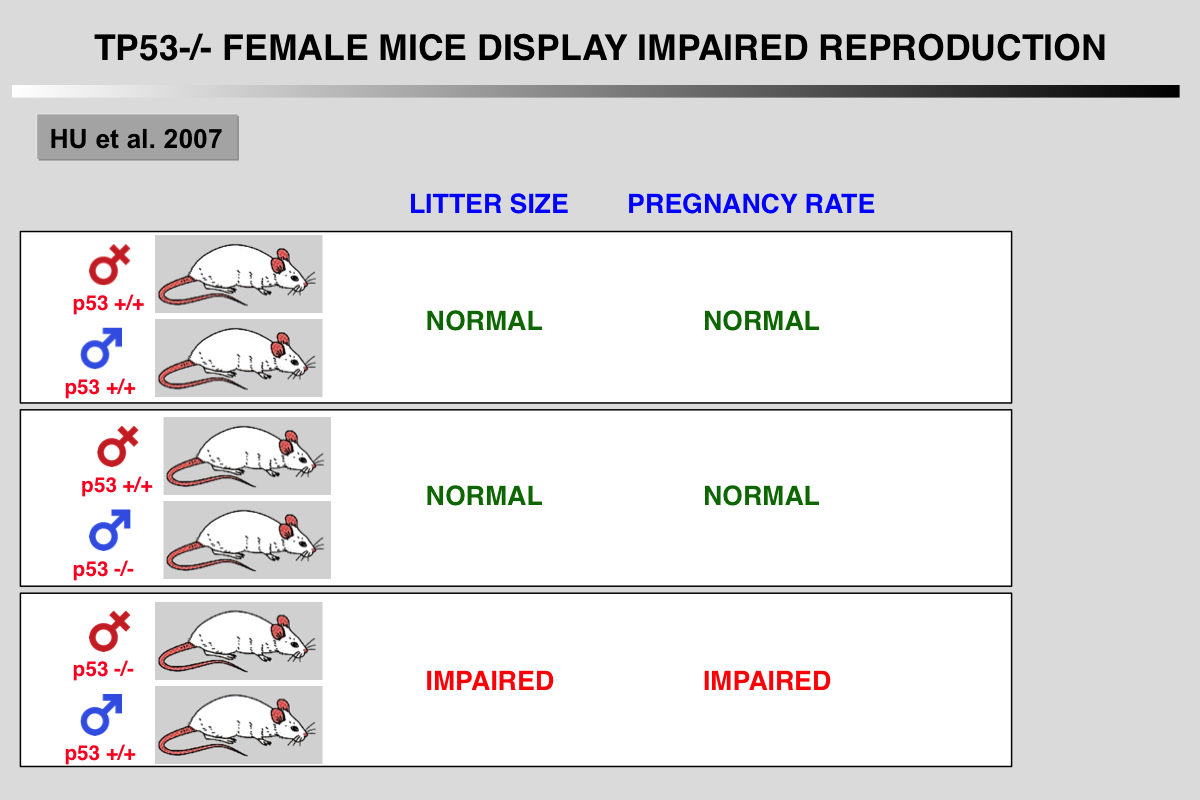
Hu, W., Feng, Z., Teresky, A. K., and Levine, A. J. (2007). p53 regulates maternal reproduction through LIF. Nature 450, 721-724.
 Abstract of the paper
Abstract of the paper
Extensive studies have shown that p53 is important in tumour prevention. However, little is known about its normal physiological function. Here we show that p53 is important in reproduction, in a gender-specific manner. Significant decreases in embryonic implantation, pregnancy rate and litter size were observed in matings with p53-/- female mice but not with p53-/- male mice. The gene encoding leukaemia inhibitory factor (LIF), a cytokine critical for implantation, was identified as a p53-regulated gene that functions as the downstream mediator of this effect. p53 can regulate both basal and inducible transcription of LIF. Loss of p53 decreased both the level and function of LIF in uteri. Lower LIF levels were observed in the uteri of p53-/- mice than in those of p53+/+ mice, particularly at day 4 of pregnancy, when transiently induced high levels of LIF were crucial for embryonic implantation. This observation probably accounts for the impaired implantation of embryos in p53-/- female mice. Administration of LIF to pregnant p53-/- mice restored maternal reproduction by improving implantation. These results demonstrate a function for p53 in maternal reproduction through the regulation of LIF. Evidence is accumulating that p53 may have a similar function in humans.
References
Almog, N., and Rotter, V. (1997). Involvement of p53 in cell differentiation and development. Biochim Biophys Acta 1333, F1-27.
Armstrong, J. F., Kaufman, M. H., Harrison, D. J., and Clarke, A. R. (1995). High-frequency developmental abnormalities in p53-deficient mice. Curr Biol 5, 931-936.
Donehower, L. A., Harvey, M., Slagle, B. L., McArthur, M. J., Montgomery, C. A. J., Butel, J. S., and Bradley, A. (1992). Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356, 215-221.
Hu, W., Feng, Z., Teresky, A. K., and Levine, A. J. (2007). p53 regulates maternal reproduction through LIF. Nature 450, 721-724.
Levine, A. J., Tomasini, R., McKeon, F. D., Mak, T. W., and Melino, G. (2011). The p53 family: guardians of maternal reproduction. Nat Rev Mol Cell Biol 12, 259-265.
Molchadsky, A., Rivlin, N., Brosh, R., Rotter, V., and Sarig, R. (2010). p53 is balancing development, differentiation and de-differentiation to assure cancer prevention. Carcinogenesis 31, 1501-1508.
Nicol, C. J., Harrison, M. L., Laposa, R. R., Gimelshtein, I. L., and Wells, P. G. (1995). A teratologic suppressor role for p53 in benzo[a]pyrene-treated transgenic p53-deficient mice. Nat Genet 10, 181-187.
Norimura, T., Nomoto, S., Katsuki, M., Gondo, Y., and Kondo, S. (1996). p53-dependent apoptosis suppresses radiation-induced teratogenesis. Nat Med 2, 577-580.
Harvey, M., McArthur, M. J., Montgomery, C. A. J., Bradley, A., and Donehower, L. A. (1993). Genetic background alters the spectrum of tumors that develop in p53-deficient mice. FASEB J 7, 938-943.
Rotter, V., Schwartz, D., Almon, E., Goldfinger, N., Kapon, A., Meshorer, A., Donehower, L. A., and Levine, A. J. (1993). Mice with reduced levels of p53 protein exhibit the testicular giant-cell degenerative syndrome. Proc Natl Acad Sci U S A 90, 9075-9079.
Sah VP, Attardi LD, Mulligan GJ, Williams BO, Bronson RT and Jacks T (1995) A subset of p53-deficient embryos exhibit exencephaly. Nat Genet 10: 175-80.
Wallingford, J. B., Seufert, D. W., Virta, V. C., and Vize, P. D. (1997). p53 activity is essential for normal development in Xenopus. Curr Biol 7, 747-757.
Wubah, J. A., Ibrahim, M. M., Gao, X., Nguyen, D., Pisano, M. M., and Knudsen, T. B. (1996). Teratogen-induced eye defects mediated by p53-dependent apoptosis. Curr Biol 6, 60-69.